- VetConsultPlus
- Informational portal
- Veterinary drugs
- Zoletil for injections! Highly effective animal anesthesia
- This article contains information regarding Zoletil-100 injection for veterinary use.
- Information provided includes the following:
- Zoletil for injection indications for veterinary medicine
- Warnings and precautions regarding Zoletil anesthesia
- Information on the use and dosage of Zoletil for injection
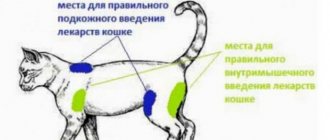
- Zoletil for injection. This drug applies to the following species: Cats, Dogs
- Company: Virbac
- Active ingredient: Tiletamine and Zolazepam
- For intramuscular and cautious intravenous use in dogs and cats only
Description
ZOLETIL® for injection (tiletamine and zolazepam) is a non-narcotic, non-barbitural injection anesthetic for dogs and cats. Chemically, ZOLETIL for injection is a combination of equal parts by weight of the base:
- tiletamine - a dissociative anesthetic, an arylaminocycloalkanone derivative
- Zolazepam is a non-phenothiazinediazepinone with minor tranquilizer properties.
Zoletil for animal anesthesia is supplied in sterile vials with lyophilisate. Addition of 5 ml of diluent gives a solution containing the equivalent of 50 mg of tiletamine base, 50 mg of zolazepam base and 57.7 mg of mannitol per milliliter. This solution has a pH of 2 to 3.5 and is recommended for deep intramuscular injections.
Release form
The drug is a transparent liquid of light yellow color. The antimicrobial agent includes the antibiotic enrofloxacin, which is a group of fluoroquinolones, as well as excipients. The following drugs are in demand:
- Enrofloxacin 100 for oral use.
- Enrofloxacin 50 for parenteral use.
- Enroflox 10. Available in oral and parenteral forms.
Preparations for internal use are packaged in plastic containers of 1 liter, injections - in glass bottles of 100 ml. Enroflox is produced by the Spanish company, Enrofloxacin by the Chinese company.
recommended articles:
The drug inhibits DNA synthesis of gram-positive and gram-negative microorganisms. It is well absorbed from the intestines and penetrates all tissues except the brain.
Enrofloxacin 100
pharmachologic effect
ZOLETIL - anesthesia for injections
is a fast-acting anesthetic combination of tiletamine hydrochloride and zolazepam hydrochloride. Tiletamine is a dissociative anesthetic, the pharmacological action of which is characterized by deep analgesia, normal pharyngeal-laryngeal reflexes and cataleptoid anesthesia. The state of anesthesia produced does not fit into the generally accepted classification of stages of anesthesia, but instead ZOLETIL - 100 creates a state of unconsciousness, which is called "dissociative" anesthesia, since it appears to selectively interrupt the association pathways to the brain and provide sensory blockade. Cranial and spinal reflexes remain active; however, these reflexes should not be confused with inadequate anesthesia. Analgesia results from the apparent selective interruption of sensory input to the brain and usually persists after the anesthetic effect has subsided.
Defensive reflexes such as coughing and swallowing are maintained under tiletamine anesthesia. Other reflexes, such as corneal and pedal reflexes, persist during tiletamine anesthesia and should not be used as a criterion for assessing the depth of anesthesia. The eyes usually remain open with the pupils dilated. It is suggested that a moisturizing ophthalmic ointment should be applied to the cornea if anesthesia is to be prolonged.
Tiletamine hydrochloride, used alone in veterinary medicine, does not provide sufficient muscle relaxation during abdominal surgery. In combination with zolazepam, good muscle relaxation is observed in animals, usually achieved during the deep surgical anesthesia phase.
After a single deep intramuscular injection of NARKOZA ZOLETIL to cats and dogs, the onset of the anesthetic effect usually occurs within 5-12 minutes. Muscle relaxation is optimal for approximately the first 20-25 minutes after administration of ZOLETIL injection, and then decreases. Recovery depends on the age and physical condition of the animal and the dose of ZOLETIL 100
, but usually takes several hours. The duration of anesthesia is prolonged by repeated injections, especially in cats.
Repeated doses increase the duration of the anesthesia effect of ZOLETIL, but cannot further reduce muscle tone. The quality of anesthesia with repeated doses varies because the ratio of the two components in the animal's body changes with each injection. This is due to the difference in the rate of metabolism and elimination of the two components. The quality of anesthesia will be improved and more predictable if the entire dose is given in one injection rather than in multiple doses. The best method for assessing the depth of anesthesia when using ZOLETIL for injection anesthesia is to monitor the patient for a conscious response to nociceptive stimuli.
Excessive salivation may occur during the use of ZOLETIL for injection anesthesia in animals. Ptyalism (salivation) can be controlled in dogs and cats by giving atropine sulfate (not indicated when alpha2-agonists such as xylazine are used concomitantly with zoletil), at a dose of 0.04 mg/kg body weight, as a concomitant medication. Excessive swallowing, reflex action and accumulation of saliva can cause vomiting and vomiting.
Zoletil have a wider therapeutic spectrum in cats than in dogs.
The dogs survived repeated dosing regimens of 30 mg/kg (the maximum safe dose) for eight consecutive days. This is approximately twice the maximum recommended therapeutic dose. The cats tolerated dosing regimens up to 72 mg/kg (maximum safe dose) on alternating days over seven episodes. This is 4.6 times the maximum recommended therapeutic dose for cats. However, these reports should not preclude careful anesthesia techniques in animals. Some degree of tolerance to zoletil has been reported in animals. This tolerance appears to be species dependent.
Cats: In cats, the duration of action of zoletil anesthesia exceeds the duration of action of tiletamine, so that as the animals recover, the degree of tranquilization is higher than with anesthesia. During the first hour after injection, a slight decrease in blood pressure is observed. Heart rate and electrocardiogram readings are not affected by tiletamine and zolazepam. Arterial pO2 levels decrease three minutes after injection but usually return to normal within 15 to 35 minutes.
Dogs: In dogs, the duration of action of tiletamine is longer than that of zolazepam, so in this species the degree of tranquilization is less than that of analgesia. The overall effect of tiletamine and zolazepam is shorter in dogs than in cats.
After administration of tiletamine and zolazepam (zoletil 100 mg)
Dogs show marked, sustained tachycardia within two minutes of intramuscular administration of 10 or 20 mg/kg tiletamine and zolazepam. Stroke volume decreases proportionally to the increase in contractile velocity at the 10 mg/kg dose, with little change in net cardiac output. There is an initial increase in systolic blood pressure with a slight drop in pressure over five minutes. Systolic blood pressure remains at this reduced level throughout the duration of the anesthetic. Diastolic pressure increases during the same period. After zoletil doses exceed 20 mg/kg in dogs, the relationship between stroke volume and heart rate is disproportionate, resulting in a significant decrease in cardiac output. Contractility and mean arterial pressure are reduced, indicating direct myocardial depression. Ventricular function is adequate. During surgical procedures, tachycardia and hypertension may occur, which may be caused by a sympathetic response to painful stimuli. Under anesthesia with zoletil, adrenaline is noticeably less arrhythmogenic in animals than in animals under anesthesia with halothane.
During zoletil anesthesia, the guarantee of an open airway is greatly improved by maintaining the pharyngolaryngeal reflexes.
During the first 15 minutes after intramuscular administration of 20 mg/kg zoletil, respiratory rate doubles, while tidal volume decreases to less than half of control values. Arterial pO2 levels also decrease. This can be confirmed by hypoxemia and cyanosis. Pulmonary function usually returns to normal within 35 minutes after administration of zoletil.
Contraindications for the use of zoletil in animals
The use of zoletil is contraindicated in dogs and cats with pancreatic diseases. Tiletamine and zolazepam are excreted primarily by the kidneys. Pre-existing renal pathology or impaired renal function can be expected to lead to an increase in the duration of anesthesia. Zoletil 100 should not be used in dogs and cats with severe cardiac or pulmonary dysfunction. Because the teratogenic potential of zoletil is unknown, it should not be used in pregnant bitches at any stage of pregnancy. In addition, the study showed that zoletil crosses the placental barrier in animals and causes respiratory depression in the newborn; therefore, its use for cesarean section is contraindicated (not recommended).
Contraindications and side effects of the drug
The manufacturer notes that Zoletil 100 is contraindicated for use in the following situations:
- diseases associated with the pancreas;
- hypertension;
- severe respiratory dysfunction;
- problems with the heart or blood vessels.
During pregnancy and when kidney disease is detected, the dosage of the drug is reduced.
Side effects may include:
- intense salivation;
- temporary cessation of respiratory movements;
- vomit;
- spasm of the bronchial muscles;
- involuntary muscle twitching or spasms;
- high blood pressure.
The above side effects can be eliminated with the help of ventilation and symptomatic therapy.
The main advantage of the anesthetic Zoletil 100 is that the active substances do not accumulate in the body of animals, after anesthesia there is a quick and quiet restoration of all functions, and the risk of overdose is minimized.
Warnings
ZOLETIL IS INTENDED FOR USE ONLY IN DOGS AND CATS. The main route of excretion for both components in cats is urine; therefore, zoletil is not recommended for use in animals suffering from renal failure.
Studies in dogs have shown extensive biotransformation of both components, with less than 4% of the dose excreted unchanged in the urine.
The safety of using zoletil in pregnant animals or during breeding has not been established. Tiletamine and zolazepam cross the placental barrier and cause respiratory depression in the newborn. Phenothiazine derivatives should not be used with zoletil 100 because the combination causes respiratory and myocardial depression, hypotension and hypothermia. Pulmonary edema has been reported to occur in cats with the use of zoletil. Signs and symptoms include shortness of breath, lethargy, anorexia and abnormal behavior. Deaths have been reported in cases of severe pulmonary edema. Cats should be closely monitored for any signs and symptoms that may indicate pulmonary edema so that appropriate therapy can be initiated. It is recommended to conduct a screening test before anesthesia in cats. And within 30 days after anesthesia with Zoletil, owners are advised to count their breathing rate during sleep. If this indicator is more than 27 times per minute, this indicates the onset of pulmonary edema and it is necessary to take the patient to a veterinary clinic.
PERSONAL PREVENTION MEASURES
When working with Zoletil®50 and 100, you should follow the general rules of personal hygiene and safety precautions provided for when working with medications. People with hypersensitivity to the components of the drug should avoid direct contact with Zoletil® 50 and 100. If the drug accidentally comes into contact with the skin or mucous membranes (it must be washed off immediately with running water and soap.
If allergic reactions occur and/or if the drug accidentally enters the human body, you should immediately contact a medical facility (bring with you the instructions for use of the drug or the label). Empty drug bottles must not be used for household purposes; They must be disposed of with household waste.
Organization-;
Icrc avenue 2065 M – LID, Carros, France 06511. READ Worms in cats - photos with names, description
The instructions have been developed; 1 SGS avenue 2065 M – LID, Carros, France 06511. With the approval of this instruction, the instructions for use of 3oletil®50 and 100, approved by Rosselkhoznadzor on October 20, 2008, become invalid. Recommended for registration in the Russian Federation by the Federal State Budgetary Institution "VGNKI".
Registration certificate number PVI-3-1.9/01425
Precautions when working with the anesthetic drug "Zoletil"
The dosage of zoletil should be reduced in older dogs and cats, in animals
in a weakened state and in animals with impaired renal function. Body temperature should be monitored and supplemental rewarming may be required to control hypothermia. As with the use of other anesthetics, it is prudent to consider hemostasis during any surgical procedure.
During anesthesia with zoletil, athetoid movement may occur.
This athetosis should not be confused with a lack of anesthesia and does not indicate a lack of pain relief. Do not give additional anesthesia in an attempt to reverse athetoid movement. Attempts to eliminate athetoid movements with additional doses of zoletil may lead to an overdose of the anesthetic. Zoletil does not eliminate laryngeal, pharyngeal, pennate, palpebral and pedal reflexes and may not be suitable as the sole anesthetic for surgical procedures in these areas.
Endotracheal tubes are poorly tolerated due to zoletil anesthesia in cats and their use may result in respiratory compromise. Once the tube is removed, normal breathing should resume. It is necessary to include propofol in the combination of anesthetics, which significantly improves the process of placing an endotracheal tube.
Stimulation of the nervous system during surgical procedures helps maintain adequate ventilation. The anesthetized patient should be monitored throughout the procedure and if cardiopulmonary problems do occur, measures should be taken to ensure that alveolar ventilation and cardiovascular function are maintained in the animal.
The eyes usually remain open with dilated pupils. The use of a moisturizing ophthalmic ointment is advisable to protect the cornea from drying out. A study showed that concomitant use of chloramphenicol will prolong the duration of anesthesia in cats.
Atropine (0.04 mg/kg) should be used to control ptyalism; atropine is not indicated when combining zoletil with xylazine (medetomidine, dexmedetomidine).
Adverse reactions to the administration of zoletil to animals
Respiratory depression in animals may occur after taking high doses of zoletil. If at any time respiration becomes excessively suppressed and the animal becomes cyanotic, resuscitation measures should be initiated immediately. Adequate ventilation using oxygen or room air is recommended as a resuscitation measure.
Adverse reactions such as vomiting during induction, excessive salivation, transient apnea, vocalization, unstable and prolonged recovery, excessive tracheal and bronchial secretions when atropine sulfate was not administered before anesthesia, involuntary muscle twitching, hypertonicity, cyanosis, cardiac arrest have been reported. , pulmonary edema and muscle stiffness during surgery. Central nervous system stimulation (excitation) and seizures have also been reported. Tachycardia often occurs, especially in dogs. This increase in heart rate usually lasts about 30 minutes. Hypertension or hypotension may also occur. Dogs often experience insufficient anesthesia when using Zoletil alone.
Deaths have been reported in dogs and cats following administration of zoletil.
Application and dosage of zoletil in animals
ZOLETIL® for anesthesia is well tolerated by dogs and cats and should be administered by deep intramuscular injection in prescribed doses. In higher doses, recovery is usually prolonged.
There may be pain during injection. This is especially common in cats.
Fasting before the introduction of general anesthesia with ZOLETIL for injection is not mandatory. However, when preparing for a planned operation, it is recommended not to eat for at least 12 hours before the administration of ZOLETIL for anesthesia.
As with other injectable anesthetics, individual response to anesthesia with ZOLETIL varies somewhat depending on the dose, the general physical condition and age of the patient and the duration of the surgical procedure. Therefore, recommendations for dosage regimens cannot be absolutely fixed. Specific dosage requirements should be determined by evaluating the medical condition of the patient and the procedure to be performed.
If adequate anesthesia is not provided according to the recommended dosage regimen, additional anesthesia or another agent is indicated. This includes the use of barbiturates and gaseous anesthetics. When used simultaneously with ZOLETIL, the dosage of these drugs should be reduced.
Atropine sulfate at a dose of 0.04 mg/kg should be used as a concomitant medication to control ptyalism.
Instructions for use of Zoletil 100 as an anesthetic for animals
Treating animals sometimes requires surgery. For pain relief, substances are used that put patients into medicated sleep. The most common is Zoletil 100. It is used for anesthesia of pets (dogs, cats) and some species of wild animals.
Before using the drug, you should study the instructions for it, which describe general information, composition, properties, method of use, contraindications and side effects.
Zoletil is a modern veterinary drug from the group of dissociative anesthetics, used for general anesthesia of animals in clinics. Manufacturer: French company Virbac Sante Animale. The product is approved for use and is not a psychotropic substance. It can be used to anesthetize pregnant and newborn animals.
Composition of Zoletil 100:
- The main substances are tiletamine hydrochloride (250 mg), zolazepam hydrochloride (250 mg).
- Auxiliary components: lactose or glucose, sodium sulfate.
Release form: lyophilized powder for injection, white or with a yellowish tint. It comes with water for preparing the solution. The packaging contains glass bottles sealed with stoppers and aluminum caps.
Zoletil anesthesia in dogs
- In healthy dogs, an initial intramuscular anesthesia dose of 6.6 to 9.9 mg/kg ZOLETIL is recommended for diagnostic purposes;
- 9.9 to 13.2 mg/kg for minor short-term procedures such as treatment of cuts and wounds, castration, and other procedures requiring mild to moderate pain relief. When additional doses of ZOLETIL are required for anesthesia, such individual additional doses should be less than the initial dose, and the total dose prescribed (initial dose plus additional dose or doses) should not exceed 26.4 mg/kg.
- The maximum safe dose of zoletil is 29.92 mg/kg.
- The results of ZOLETIL for injection anesthesia in dogs will be more satisfactory if procedures are completed within one hour and if procedures can be completed after a single dose. To maintain at least a 2-fold safety margin in dogs, use of this product is limited to procedures requiring low doses. Studies show that there are differences in response to different doses of ZOLETIL injection and that low doses do not provide adequate levels of anesthesia, and in some cases do not provide adequate pain relief for major procedures. Intravenous dosages of zoletil are 5 times less than those recommended in the instructions. The drug is administered intravenously by titration. The duration of the effect is reduced.
Zoletil anesthesia in cats
In healthy cats, the starting dose of ZOLETIL® for injection is 9.7 to 11.9 mg/kg
recommended for procedures such as dentistry, abscess treatment, foreign body removal and related types of surgery;
10.6 to 12.5 mg/kg) for minor procedures requiring mild to moderate analgesia, such as repair of tissue tears, castration, and other short procedures. Initial doses of 14.3 to 15.8 mg/kg
are recommended for ovariohysterectomy and onychectomy. When additional doses of ZOLETIL injection are required, such individual additional doses should be administered in increments smaller than the initial dose, and the total dose given (initial dose plus additional doses) should not exceed the maximum safe dose of 72 mg/kg. The drug is administered intravenously by titration in lower doses of 10-20% of those recommended in the instructions. The duration of the effect is reduced. A standard solution of zoletil in a dose of 0.1 ml is drawn into an insulin syringe and adjusted to 1 ml with saline. The indicated solution is titrated into intravenous induction and maintenance during ovariohysterectomy in cats. This syringe is enough for standard sterilization. Premedication is carried out with gabapentin and dexmedetomidine.
How is the drug supplied for veterinary use?
ZOLETIL for animal anesthesia is available in individual bottles with 5 ml of solution after reconstitution.
Addition of 5 ml of diluent gives a solution containing the equivalent of 50 mg of tiletamine base, 50 mg of zolazepam base and 57.7 mg of mannitol per milliliter.
5 ml bottle - 100 mg/ml (when reconstituted).
Store at controlled room temperature between 20 and 25°C.
The color of zoletil solution can vary from colorless to light amber.
COMPOSITION GOLED
As active ingredients, 1 bottle of Zoletil®50 contains: 125 mg of tiletamine base (equivalent to 145.40 mg of tiletamine hydrochloride) and 125 mg of zolazepam base (equivalent to 140.52 zolazepam hydrochloride), as well as excipients: sodium sulfate - 9.08 mg and lactose - 400 mg; 1 bottle of Zoletil®100 contains as active ingredients: 250 mg of tiletamine base (equivalent to 290.85 mg of tiletamine hydrochloride) and 250 mg of zolazepam base (equivalent to 281.00 mg of zolazepam hydrochloride), as well as excipients: sodium sulfate - 18, 15 mg and lactose - 400 mg. Zoletil®50 and 100 are packaged complete with a solvent – water for injection (5 ml). In appearance, the drug is a compact mass of white or light yellow color; solvent is a colorless transparent liquid. The drug is released in sterile packages of 695 mg (Zoletil®50) and 990 mg (Zoletil®100) in hermetically sealed glass bottles. The solvent is released packaged in 5 ml glass bottles of appropriate capacity. Zoletil®50 and 100 are packaged in cardboard boxes containing 1 or 10 bottles of the drug and 1 or 10 bottles of solvent.
Store in sealed manufacturer's packaging, in a dry place, protected from direct sunlight at a temperature of 5°C to 25°C. The shelf life of the drug, subject to storage conditions in closed packaging, is 2 years from the date of production. After dissolving the drug, the finished solution can be stored for 48 hours at a temperature of 20°C and for 8 days at a temperature of 4°C. The drug must not be used for